
But as the chemical properties repeated, he began a new row. In his research journal, Mendeleev wrote that the idea for this table came to him in a dream. Such patterns allowed scientists to anticipate whether or how different types of elements would likely combine. Certain elements prefer to react, becoming positively charged. As elements get larger, some of their properties eventually repeat. He was one of the early scientists who realized that chemistry has repeating patterns. These supernovas forcefully slammed smaller elements together.įor his 1869 periodic table, Mendeleev arranged the elements in order of ascending mass. Heavy atomic nuclei formed as massive, dying stars exploded. Making elements larger than iron required even more cosmic firepower. Those stellar forges also formed the oxygen that we need to breathe. They included carbon, an element essential for all life as we know it. This slowly forged larger and heavier elements. In the center of these stars, intense pressures fused atomic nuclei - the centers of the atoms - creating larger nuclei.
Eventually this would create dense, fiery hot furnaces that we know of as stars. Gravity brought these atoms together in ever larger amounts. Just after the Big Bang, the universe was made up of only hydrogen and helium - the two lightest elements. In 2019, the world is celebrating the periodic table in all its forms and how it helps organize and make sense of the building blocks of our universe. Those less traditional periodic tables provide ways not only to highlight some of chemistry’s quirks, he says, but also to bring them into better focus. He’s a chemist at Le Moyne College in Syracuse, N.Y. “Alternate forms are useful because of the different aspects of the science that they illustrate,” notes Carmen Giunta. Scientists and teachers in other fields developed others. Scientists have built many, some with widely varying shapes. It even helps them predict how new materials will behave.ĭmitri Mendeleev/Science History Instituteīut Mendeleev’s well-known chart is far from the only periodic table. It also helps them understand how life works. Understanding those relationships helps chemists create new compounds.

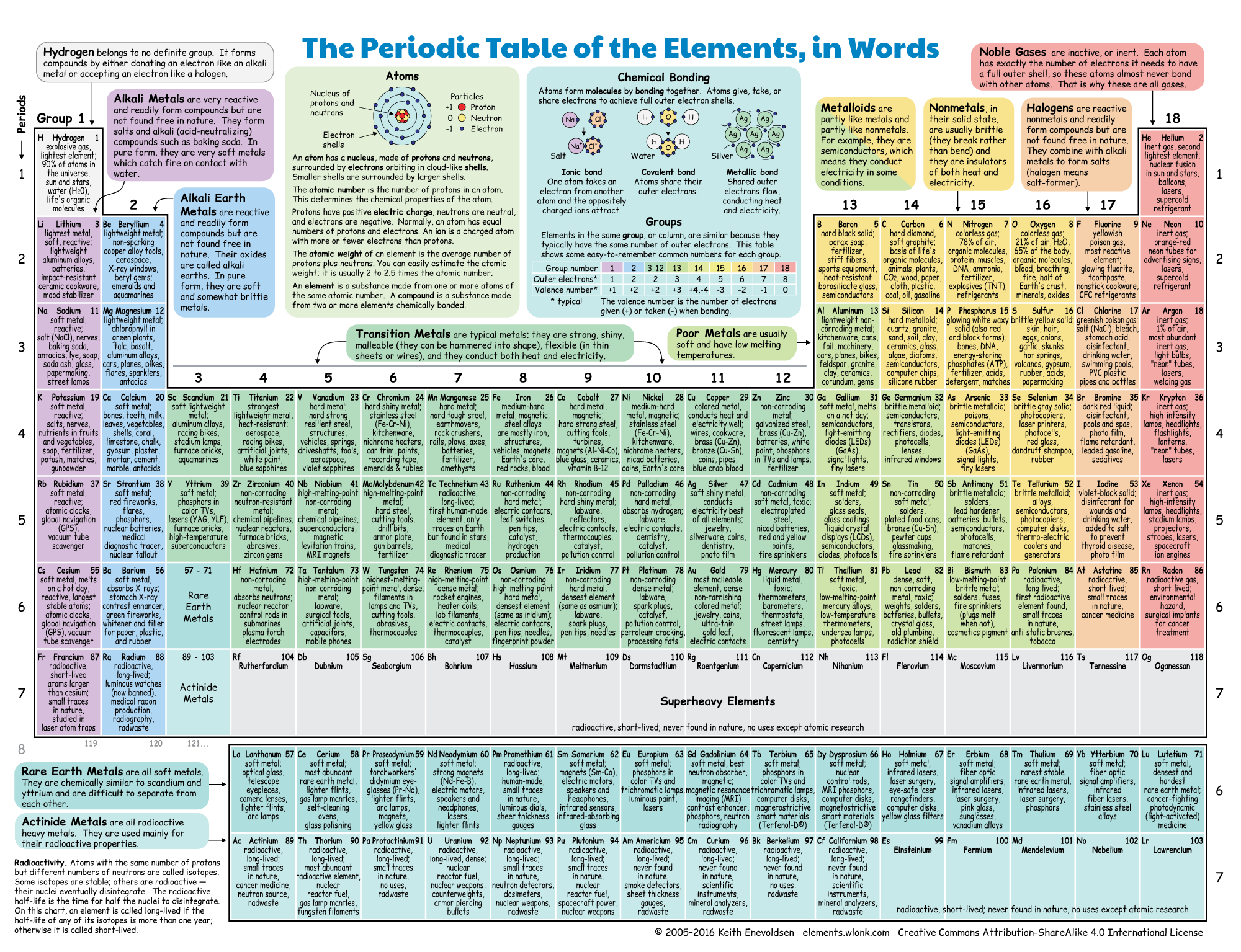
How the rows and columns on this table line up points to shared traits between groups of related elements. These patterns link elements with similar chemical behaviors and help to tell chemists how atoms react to form molecules. It holds that shared traits among chemical elements repeat in regular patterns as elements get larger.

The rows and columns on the periodic table map the so-called periodic law. Their atoms knit together to form literally everything - us, the air we breathe, the organisms that share our world and every other molecule of gas or bit of mass found throughout our universe. Yet even today, this chart helps scientists make sense of the atoms and molecules that make up our universe.Įlements are the building blocks of all matter. Petersburg, came up with an early version. Dmitri Mendeleev (MEN-duh-LAY-ev), a Russian scientist working in St. This chart - an icon of chemistry - is known as the Periodic Table of the Elements. Hanging on the wall, the squares look like a jagged wall of alphabet blocks.


 0 kommentar(er)
0 kommentar(er)
