

The trials also showed that ICS/LABA combination medicines were more effective in decreasing asthma attacks (e.g., the need to use oral corticosteroids) compared to ICS alone. The results of all trials showed that the use of LABA with ICS does not significantly increase the risk of serious asthma outcomes compared to ICS alone. Patients in all the trials were treated for 6 months to evaluate serious asthma outcomes including asthma-related death, intubation, or hospitalization. We evaluated four recently completed clinical trials involving 41,297 patients, three conducted in patients 12 years and older, and one in children 4 to 11 years. Also read the patient information leaflet that comes with every prescription. Do not stop taking your asthma medicines without first talking to your health care professional.
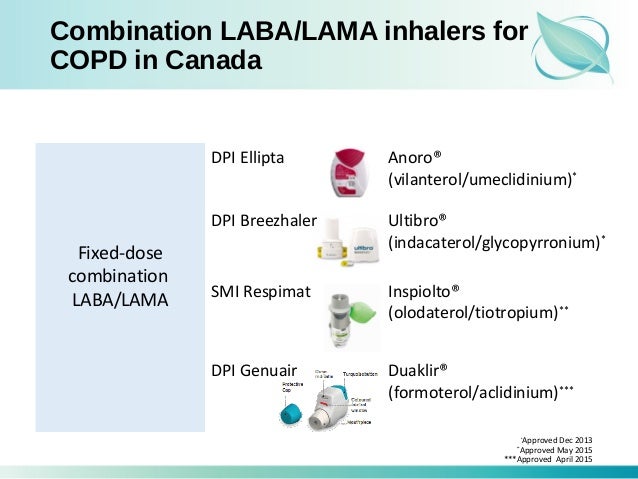
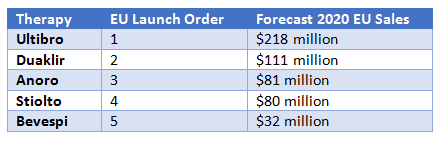
#LAMA LABA DRUG NAMES PROFESSIONAL#
Patients and parents/caregivers should talk to your health care professional if you have any questions or concerns. Health care professionals should refer to the most recently approved drug labels for recommendations on using ICS/LABA medicines (see links in Table 1). ICS/LABA medicines are marketed under several brand names, including Advair, Airduo, Breo, Dulera, and Symbicort (see Table 1). LABAs help the muscles around the airways in the lungs stay relaxed to prevent symptoms such as wheezing, coughing, chest tightness, and shortness of breath. This inflammation can lead to breathing problems.
ICS medicines help decrease inflammation in the lungs. Medicines that contain both an ICS and LABA are FDA-approved to treat both asthma and COPD.
The labels of medicines that contain both an ICS and LABA also retain a Warning and Precaution related to the increased risk of asthma-related death when LABAs are used without an ICS to treat asthma. Therefore, the Boxed Warning stating this will remain in the labels of all single-ingredient LABA medicines, which are approved to treat asthma, chronic obstructive pulmonary disease (COPD), and wheezing caused by exercise. Using LABAs alone to treat asthma without an ICS to treat lung inflammation is associated with an increased risk of asthma-related death. These trials showed that LABAs, when used with ICS, did not significantly increase the risk of asthma-related hospitalizations, the need to insert a breathing tube known as intubation, or asthma-related deaths, compared to ICS alone. A description of the four trials is now also included in the Warnings and Precautions section of the drug labels. In 2011, we required the drug companies that market LABAs to conduct these trials to evaluate the safety of LABAs when used in combination with ICS, and we reviewed the results of these recently completed trials.īased on our review, the Boxed Warning, our most prominent warning, about asthma-related death has been removed from the drug labels of medicines that contain both an ICS and LABA. Food and Drug Administration (FDA) review of four large clinical safety trials shows that treating asthma with long-acting beta agonists (LABAs) in combination with inhaled corticosteroids (ICS) does not result in significantly more serious asthma-related side effects than treatment with ICS alone.


 0 kommentar(er)
0 kommentar(er)
